
Insights+: The US FDA New Drug Approvals in March 2023
Shots:
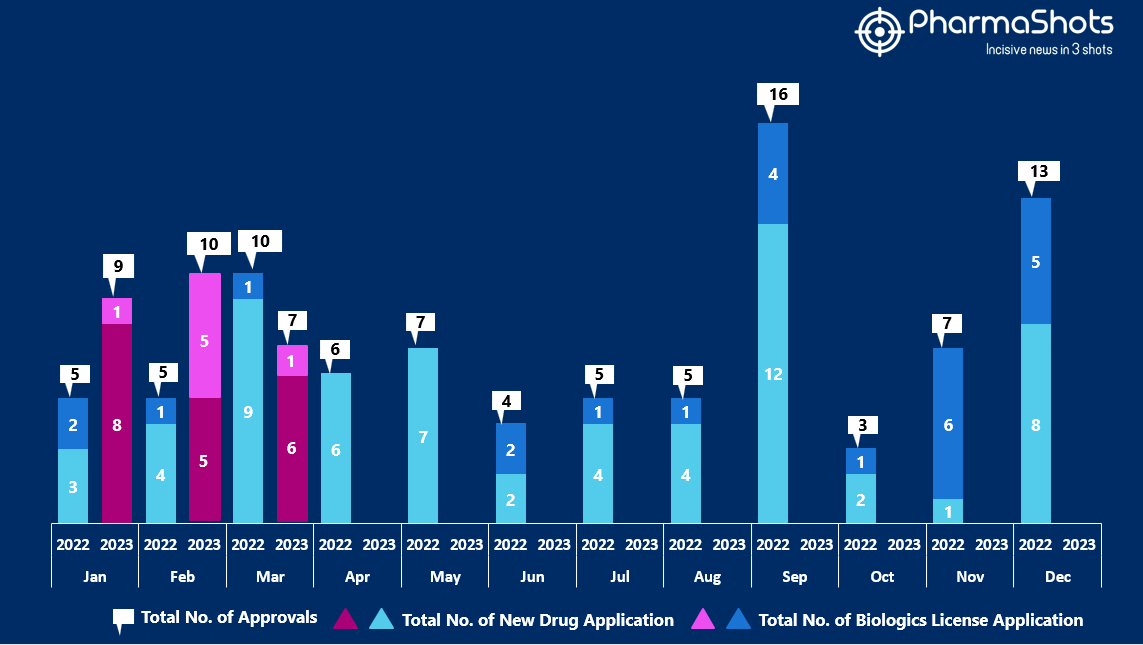
- The US FDA approved 6 NDAs and 1 BLA in March 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 26 novel products in 2023
- In March 2023, the major highlights drugs were, Tafinlar + Mekinist approval for BRAF V600E low-grade glioma, Zynyz for metastatic or recurrent locally advanced merkel cell carcinoma
- PharmaShots has compiled a list of a total of 7 new drugs approved by the US FDA in March 2023
Kevzara
Active ingredient: sarilumab Approved: March 01, 2023
Company: Regeneron and Sanofi Disease: Polymyalgia Rheumatica
- The US FDA has approved Kevzara, a human anti-IL-6Rα mAb for PMR who have had an inadequate response to CS or cannot tolerate CS taper
- The approval was based on the P-III trial (SAPHYR) evaluating Kevzara (200mg, q2w) with a 14wk. CS taper vs PBO (q2w) with a 52wk. CS taper in a ratio (1:1) in 118 patients. The trial met its 1EPs i.e., sustained remission (28% vs 10%) @52wks. Results of a sensitivity analysis excl. CRP from the sustained remission definition was consistent with the primary analysis (31.7% vs 13.8%), the incidence of TEAEs (94.9% vs 84.5%)
- The 2EPs showed the median cumulative CS dose was 777mg vs 2044mg. The companies launched the patient support program i.e., KevzaraConnect to provide access to patients for Kevzara treatment & offer support from nurses & other specialists
Pfizer’s Zavzpret (zavegepant) Receives the US FDA’s Approval for the Treatment of Migraine
Zavzpret
Active ingredient: zavegepant Approved: March 13, 2023
Company: Pfizer Disease: Migraine
- The US FDA has approved Zavzpret, the calcitonin gene-related peptide receptor antagonist nasal spray for migraine with/out aura. The therapy is expected to be available in July 2023
- The approval was based on 2 pivotal studies evaluating Zavzpret. The results showed that Zavzpret was superior to PBO on the co-primary EPs of pain freedom & freedom from most bothersome symptoms @2hrs. post-dose & was well tolerated
- The P-III study published in The Lancet Neurology found that Zavzpret was more effective than PBO across 13 of 17 prespecified secondary outcome measures incl. early time point EPs (relieve pain within 15-30 min., return to normal function in 30min.), return to normal function after 2hrs., durable efficacy EPs (sustained pain freedom, sustained pain relief from 2-48hrs.)
Daybue
Active ingredient: trofinetide Approved: March 13, 2023
Company: Pfizer Disease: Rett Syndrome
- The US FDA has approved Daybue for the treatment of Rett syndrome in adult and pediatric patients aged ≥2yrs. The product is expected to be available in the US at the end of April 2023
- The approval was based on the results from the P-III study (LAVENDER) evaluating trofinetide vs PBO in 187 female patients aged 5-20yrs. which showed an improvement on both co-primary efficacy EPs as measured by the change from baseline in RSBQ total score and the Clinical Global Impression-Improvement (CGI-I) scale score @12wks.
- Acadia & Neuren Pharmaceuticals collaborated in 2018 for the development and commercialization of trofinetide to treat Rett syndrome and other indications in North America
Tafinlar and Mekinist
Active ingredient: dabrafenib and trametinib Approved: March 17, 2023
Company: Novartis Disease: BRAF V600E Low-Grade Glioma
- The US FDA has approved Tafinlar (dabrafenib) + Mekinist (trametinib) for pediatric patients aged ≥1yr. with LGG with a BRAF V600E mutation who requires systemic therapy. The US FDA also approved liquid formulations of Tafinlar and Mekinist
- The approval was based on the results from the P-II/III trial (TADPOLE) trial evaluating Tafinlar + Mekinist (BRAF/MEK inhibitor) vs CT in 149 children and adolescent patients
- The results showed that patients experienced an improvement in ORR of 47% vs 11% and m-PFS was 20.1 vs 7.4mos. at a median follow-up of 18.9mos. The safety profile was consistent with the known safety profile in other approved indications & the results was presented at ASCO 2022
Rezzayo
Active ingredient: rezafungin Approved: March 22, 2023
Company: Cidara Therapeutics Disease: Candidemia and Invasive Candidiasis
- The US FDA has approved Rezzayo for candidemia and invasive candidiasis in adults with limited or no alternative treatment options. The approval was based on the P-III trial (ReSTORE) and supporting data from the P-II trial (STRIVE) along with an extensive non-clinical development program evaluating Rezzayo (qw)
- The studies met their 1EPs i.e., treatment with rezafungin was found to be non-inferior to caspofungin, overall rates of AEs and serious AEs were comparable, treatment discontinuation due to AEs were also similar for Rezzayo and caspofungin
- The therapy is currently being studied for the prevention of invasive fungal diseases in adults undergoing allogeneic blood and marrow transplantation
Zynyz
Active ingredient: retifanlimab Approved: March 23, 2023
Company: Incyte Disease: Merkel Cell Carcinoma
- The US FDA has approved Zynyz for adults with metastatic or recurrent LA MCC who had not received prior systemic therapy for their advanced disease
- The approval was based on the (POD1UM-201) trial evaluating Zynyz which showed ORR (52%) in CT-naïve patients, CR (18%), and PR (34%) while DoR ranged from 1.1-24.9+ mos. in the responding patients, 76% experienced a DoR at ≥6mos. and 62% at ≥12mos. by landmark analysis
- Serious AEs in 22% of patients with Zynyz & AEs lead to permanent discontinuation (11%). The company also provides a patient support program i.e., IncyteCARES that offers access to the treatment Zynyz to eligible patients in the US incl. financial assistance, ongoing education & additional resources
Joenja
Active ingredient: leniolisib Approved: March 27, 2023
Company: Pharming Disease: Phosphoinositide 3-Kinase Delta Syndrome
- The US FDA has approved Joenja (selective PI3Kδ inhibitor) for APDS in adult & pediatric patients aged ≥12yrs. The product is expected to be available in the US in early April & will be available for shipment in mid-April
- The approval was based on the P-II/III trial evaluating Joenja (70mg, BID) vs PBO in 31 patients. The 12wk. results showed the clinical efficacy of Joenja & were significant in the co-1EPs that assessed improvement in lymphoproliferation as measured by a decrease in lymph node size & increase in naïve B cells thus reflecting the impact on immune dysregulation & normalization of immunophenotype
- The MAA of leniolisib is under the EMA’s review & opinion is expected in H2’23. The company also plans for regulatory approvals in the UK, Canada, Australia & Japan
Related Post: Insights+: The US FDA New Drug Approvals in February 2023
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














